Abstract
Introduction: End stage renal disease (ESRD) has become a universal public health problem. Chronic kidney disease (CKD) is immune deficient state. So, they may be infected with hepatitis B virus (HBV) and hepatitis C virus (HCV) during hemodialysis. This becomes an important cause of morbidity and mortality among patients of hemodialysis.
Objectives: To find out the seroprevalence and incidence rate of hepatitis B and hepatitis C infections in CKD patients on hemodialysis in a tertiary care hospital.
Material and methods: A total of 380 blood samples were collected from CKD stage V patients on hemodialysis. The serum samples tested for HBsAg and anti-HCV by Vitros ECiQ (Ortho Clinical Diagnostics) system, 3rd generation. Positive results for HBsAg & anti-HCV reactive by chemiluminescence method (VITROS ECiQ), was confirmed by enzyme-linked fluorescent immunoassay method by using mini VIDAS, BioMerieux. Results: Seroprevalence of hepatitis C virus (6.57%) is higher than hepatitis B virus (1.84%). Incidence rate of HBV is 5.2 per 1000 cases in the institution. The incidence rate of HCV is 0 per 1000 cases per year.
Discussion: In the present study, the prevalence of HBV infection in HD patients was 1.84% and the prevalence of HCV infection in HD patients was 6.57%. The variations in prevalence of HBV and HCV depend mainly on the strict adherence to universal infection control precautions. This will decrease the HBV and HCV prevalence rate among these patients.
Conclusion: Chronic renal failure (CRF) patients who undergo repeated HD are at high risk of developing HBV and HCV. Proper monitoring for the early detection is required. Blood-transfusion, duration of dialysis, vaccinations are important risk factor for infection.
Keywords: seroprevalence; hepatitis B virus; hepatitis C virus; chronic kidney disease; hemodialysis
Full Text
Introduction
End stage renal disease (ESRD) has become a universal public health problem worldwide [1-2], due to increased prevalence of diabetes mellitus [3] and hypertension [4]. In western countries, diabetes and hypertension account for over 2/3rd of the cases of chronic kidney disease (CKD) [5]. In India, diabetes and hypertension today account for 40–60% cases of CKD [6]. Among these subjects, 25–40% is likely to develop CKD and hence, the ESRD burden will rise. Conventional hemodialysis remains the most common treatment for ESRD worldwide, and is usually performed for 3–5 h, three days per week [7, 8].
CKD is defined as any abnormality of kidney structure or function, present for > 3months, with implications of health. The stages of CKD according to Kidney Disease: Improving Global Outcomes (KDIGO) guideline is described in Table 1.
Table 1: CKD classification and staging according to KDIGO guidelines.
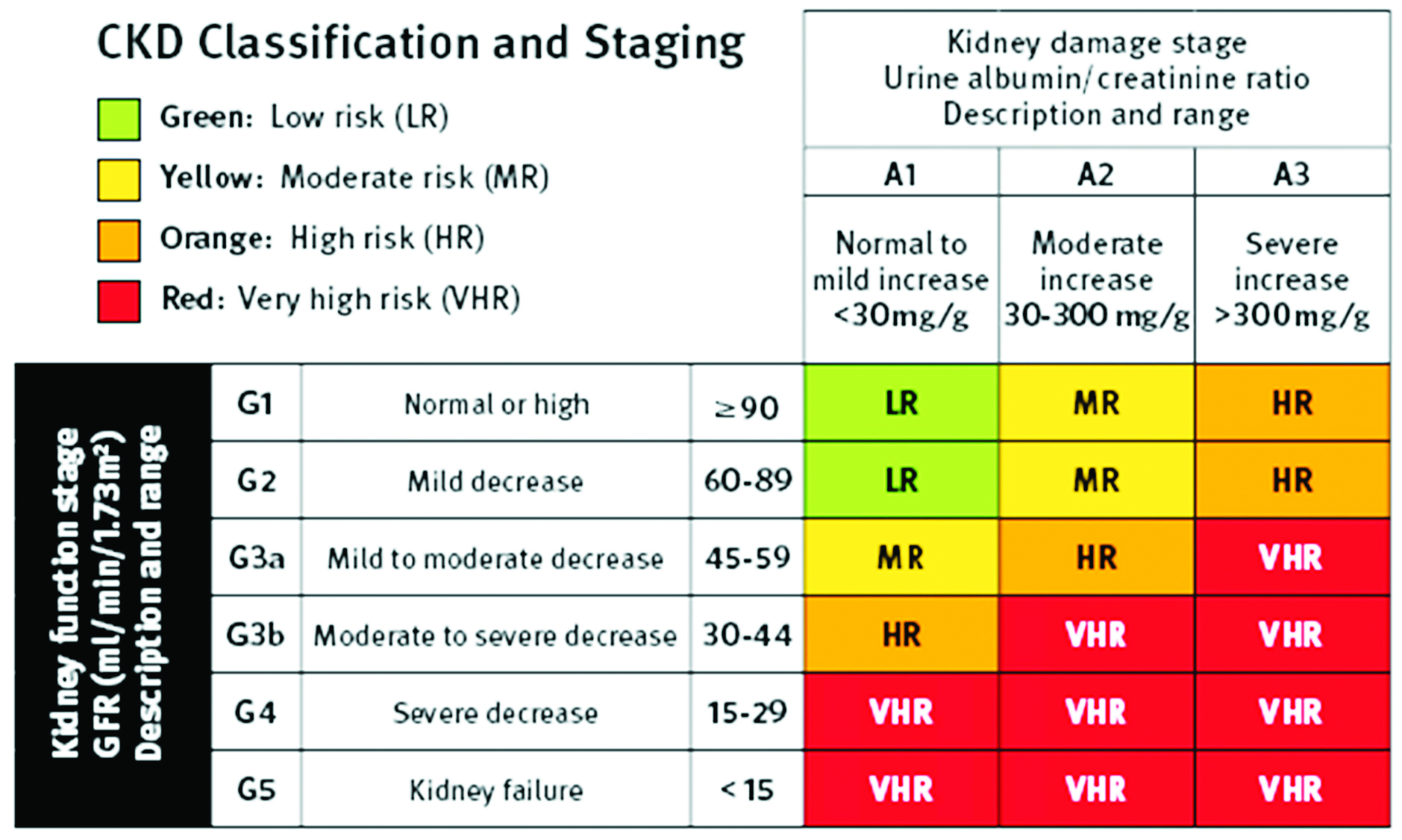
CKD is immune deficient state; hence, they may be infected with hepatitis B virus (HBV) and hepatitis C virus (HCV) during hemodialysis. Eventually, HBV and HCV infection is an important cause of morbidity and mortality among patients of hemodialysis [9].
Hepatitis C virus is more common in dialysis patients than in healthy populations and decreased survival among patients with chronic kidney disease stage V patients [10, 11]. There is large variability in prevalence among countries and within countries, among hemodialysis centers [12]. Risk factor for hepatitis B and C in dialysis patient includes the number of blood products received [13], dialysis vintage [14], the prevalence of hepatitis B and hepatitis C infection within individual dialysis unit [14] and male gender [15, 16].
In the present study, screening done to know the seroprevalence of HBV and HCV infection in all CKD stage V patients under dialysis were screened for HBV and HCV infections from September 2015 to August 2016 (one year). Since CKD stage V patients have impaired immune system, the study was emphasized to know the seroprevalence of HBV and HCV infection and to assess their risk of transmission, to calculate mean age and to find gender predominance.
To find out the seroprevalence and Incidence rate of hepatitis B and hepatitis C infections in CKD patients on hemodialysis in a tertiary care hospital. The objectives are (1) To screen HBV and HCV infection in CKD stage V patients on hemodialysis; (2) To assess the risk factors of HBV and HCV infection: role of blood transfusion, vaccination and dialysis vintage; (3) To calculate mean age and to find gender predominance.
Material and methods
The present study was conducted in the Department of Microbiology, and Dialysis unit of Nephrology, Krishna Institute of Medical Sciences, Secunderabad, India from September 2015 to August 2016 (one year). It is a prospective, observational and longitudinal study. A total of 380 blood samples were collected from CKD stage V patients on hemodialysis attending outpatient Department of Dialysis unit, Krishna Institute of Medical Sciences, Secunderabad, India. The serum samples were tested for HBsAg and anti-HCV by Vitros ECiQ (Ortho Clinical Diagnostics) system, 3rd generation. Principles of the procedure or HBsAg detection & anti-HCV detection in VITROS ECiQ (Ortho Clinical Diagnostics) is based on chemiluminescence method [17].
Inclusion criteria: All old and new cases of seronegative and seropositive (HBV and HCV) patients undergoing dialysis; patient who are transferred from other dialysis units; patients undergoing blood/blood products transfusion; patient receiving and not receiving vaccination; patient undergoing transplantation; age group: >18yrs-80yrs.
Exclusion criteria: Hepatitis A, E, D virus infected patients are not included; age group < 18 years to > 80years; pregnant women.
Statistical analysis
After taking ethical committee permission, patients were enrolled in study. Eligible patients were included into study after obtaining written informed consent. The study population was divided into groups based on incidence of hepatitis B and/or HCV or no infection. The differences between the groups for continuous variables were analyzed using independent student t-test. A p < 0.05 was considered statistically significant. After data collection and preparation of master sheet, analysis was done by using Statistical Package for Social Sciences (SPSS) version 20.0 for Windows, IBM Computers, New York, USA.
The blood samples were collected in yellow gel vacutainer by venepuncture following the standard precautions. The collected blood samples were transported to the Department of Microbiology. It was then centrifuged at 3500 rpm for 20 min and the serum gets separated. Serum samples were refrigerated (2-8°C) or stored frozen in a deep freezer (-20oC), if not tested within two days.
All 380 serum samples were tested for the seroprevalence of HBV and HCV infection by determining the presence of HBsAg and anti-HCV antibodies using a 3rd generation VITROS ECiQ (Ortho Clinical Diagnostics). Screening parameters were HBsAg and anti-HCV by Vitros ECiQ (Ortho Clinical Diagnostics) system, 3rd generation. According to Institutional policy, a repeat testing was done every three months.
When a sample is found to be positive for HBsAg & anti-HCV reactive by chemiluminescence method (VITROS ECiQ), the test result is confirmed by testing enzyme-linked fluorescent immunoassay (ELFA) method by using mini VIDAS, BioMerieux.
Results
A total of 380 cases were included in the study, maximum number of cases were in the age group between 41 and 60 years (49.5%) and least number of cases were in the age group up to 20 years (1.8%) (Table 2).
Table 2: Age wise distribution.
The Figure 1 shows mean age of HBV infection under hemodialysis is 50.14yrs and for HCV is 51.88years. The calculated p value is 0.9 by ANOVA (p > 0.05) and hence statistically not significant.
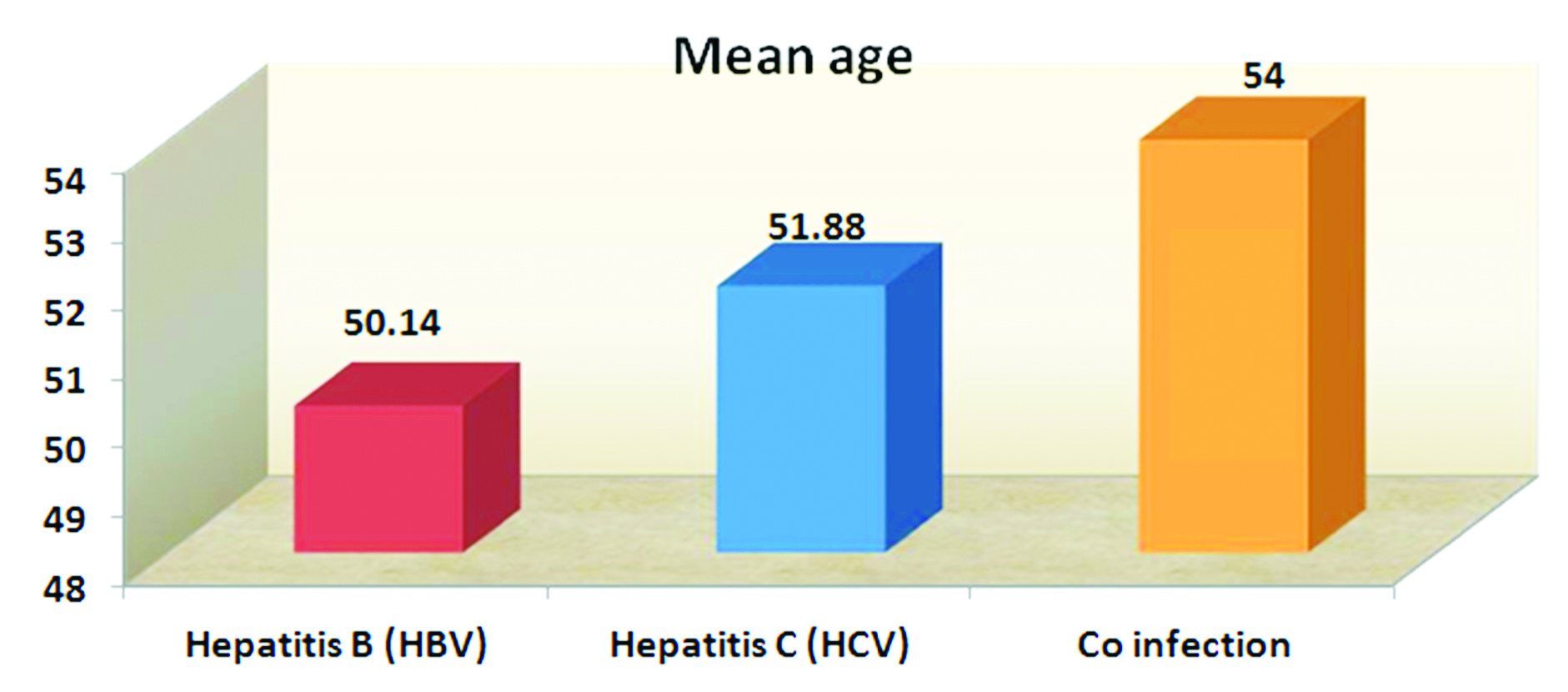
Figure 1: Association between age and seroprevalence.
The Figure 2 shows that males (n = 256; 67.36%) are more than females (n = 124; 32.63%). And also males are more prone to get infected by HBV and HCV infections. The calculated p value is 0.3 by Yates corrected chi square test (p > 0.05) and hence statistically not significant.
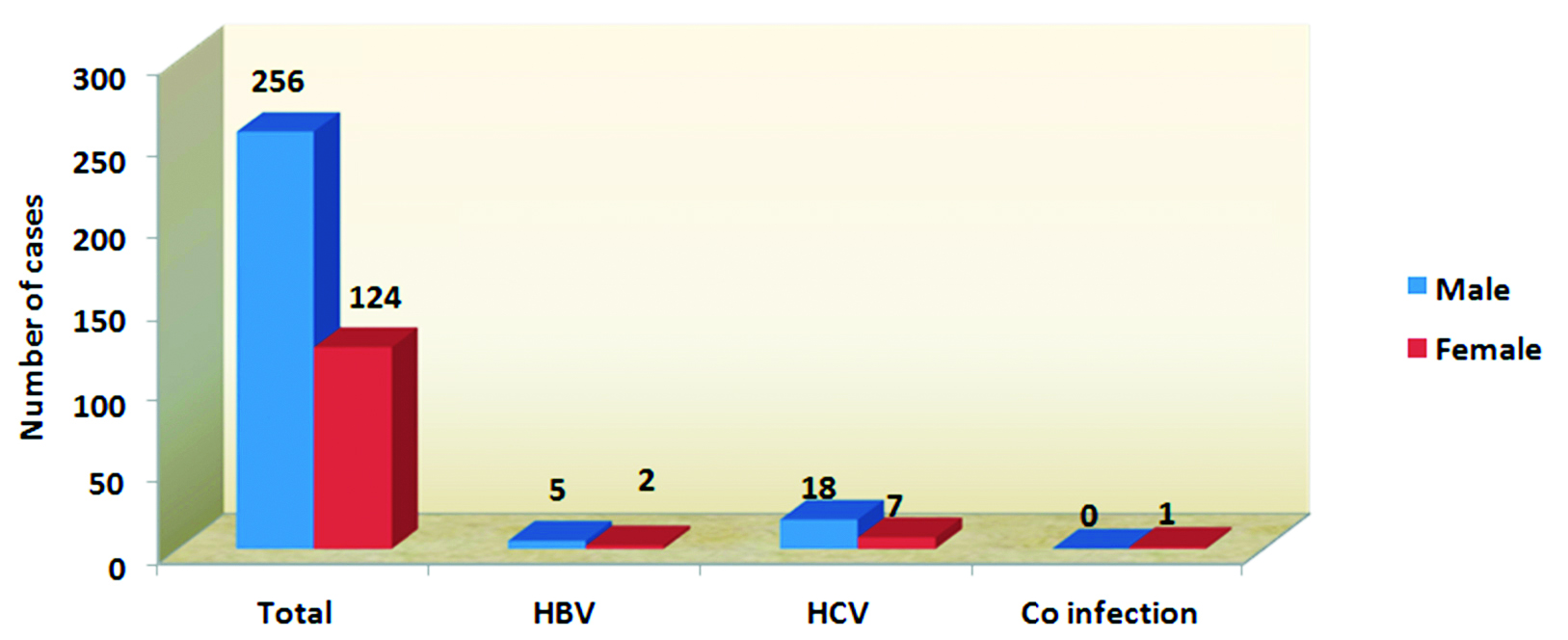
Figure 2: Association between gender and seroprevalence.
A total of 380 cases were included in the study, among them 137 were the old cases (including 5 HBV and 25 HCV positive cases) and 243 were the new cases (including 2 HBV positive cases) as shown in Table 3. Seroprevalence of hepatitis C virus (6.57%) is higher than hepatitis B virus (1.84%) (Table 4).
Table 3: Types of cases showing serological variation.
Table 4: Seroprevalence of hepatitis B virus (HBV) and hepatitis C virus (HCV) in chronic kidney disease patients on hemodialysis.
The incidence rate of HBV is 5.2 per 1000 cases in the institution. The incidence rate of HCV is 0 per 1000 cases per year described in Figure 3.
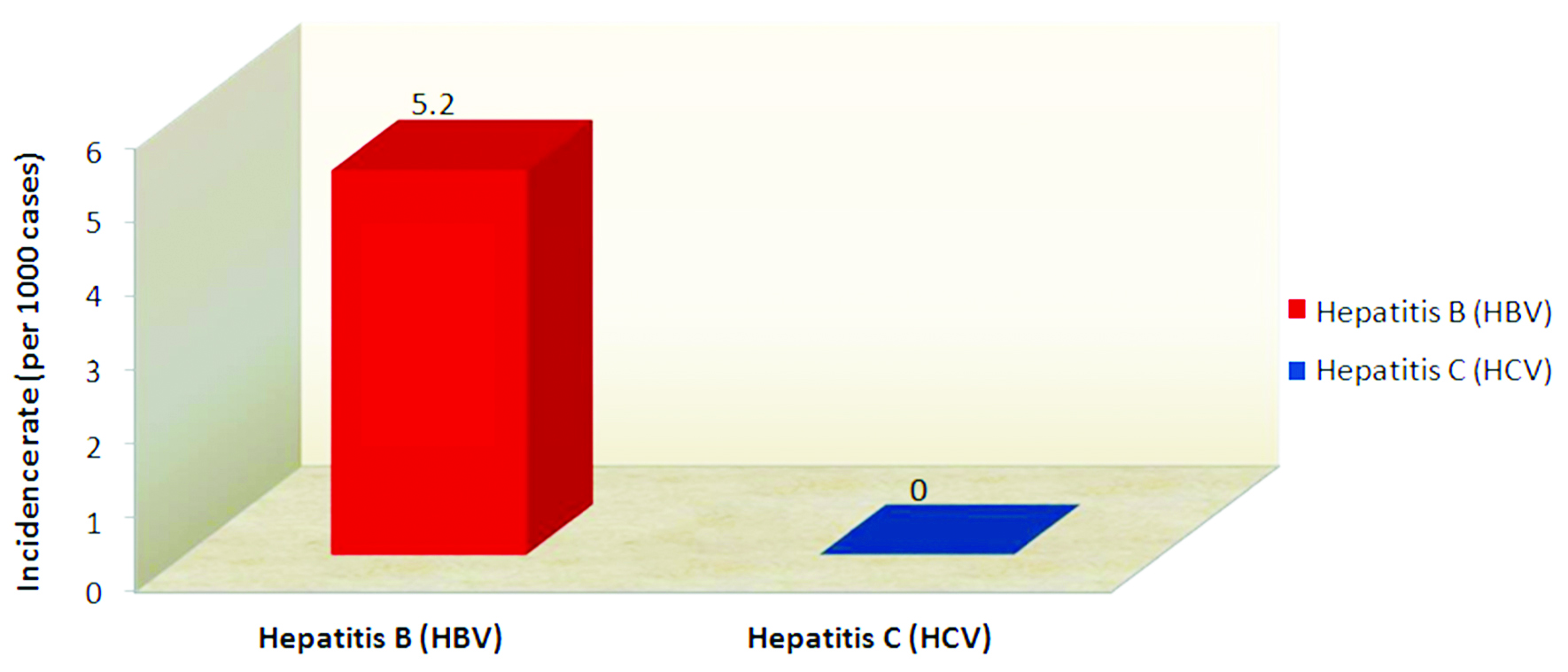
Figure 3: Incidence rate of hepatitis B virus (HBV) and hepatitis C virus (HCV) in chronic kidney disease patients on Hemodialysis.
The Figure 4 shows significant association between history of blood transfusion and HBV and HCV infections. Among all HCV positive patients, 36% patients had past history of blood transfusion. All HBV positive patients i.e. 100% of HBV infected patients, had history of previous blood transfusion. The p value is < 0.006 by Yates corrected chi square test (p < 0.05 hence statistically significant).
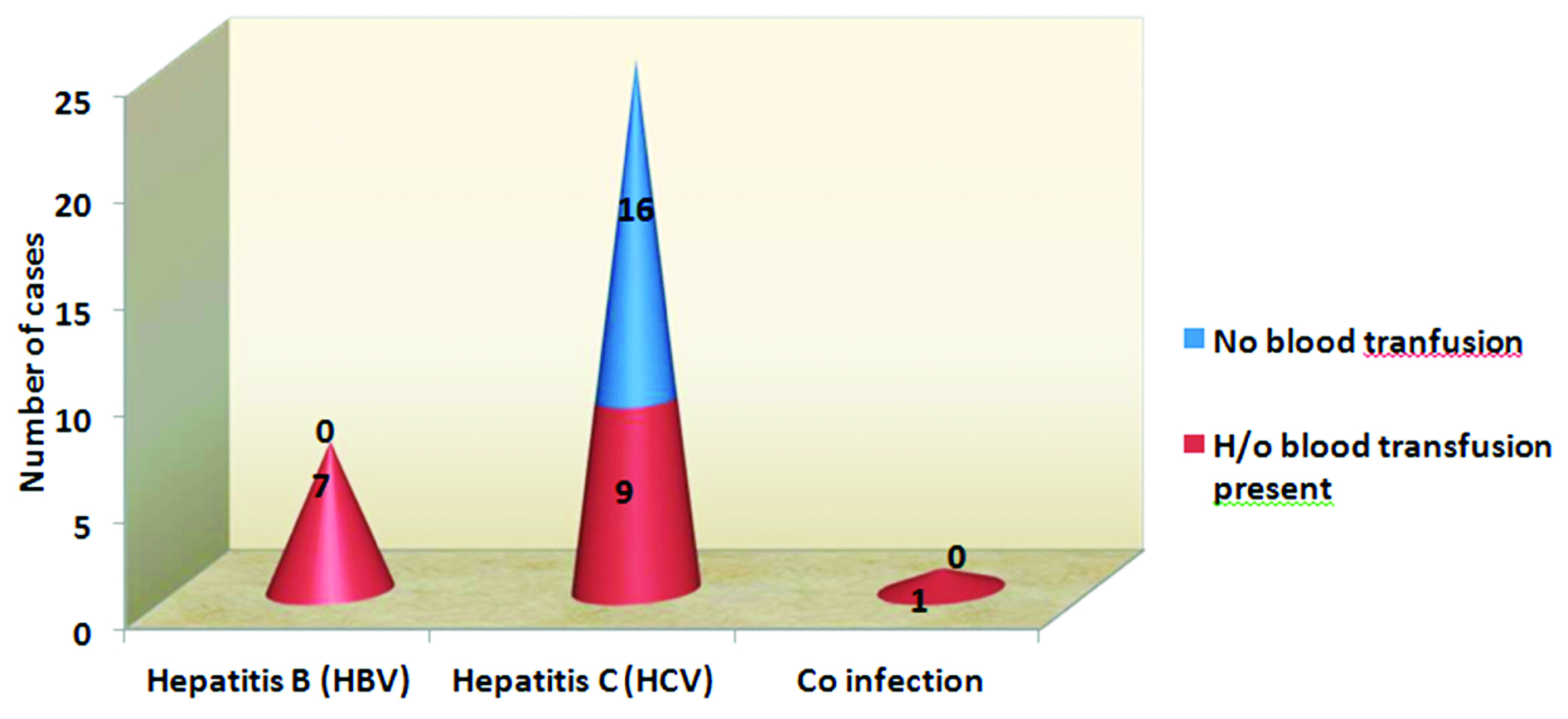
Figure 4: Association between blood transfusion and seroprevalence.
The Figure 5 shows the significant association between history of vaccination against HBV and HBV & HCV infections. The p value is < 0.04 by Yates corrected chi square test (p < 0.05 hence statistically significant). HCV infections are seen more in case of no history of vaccination against HBV.
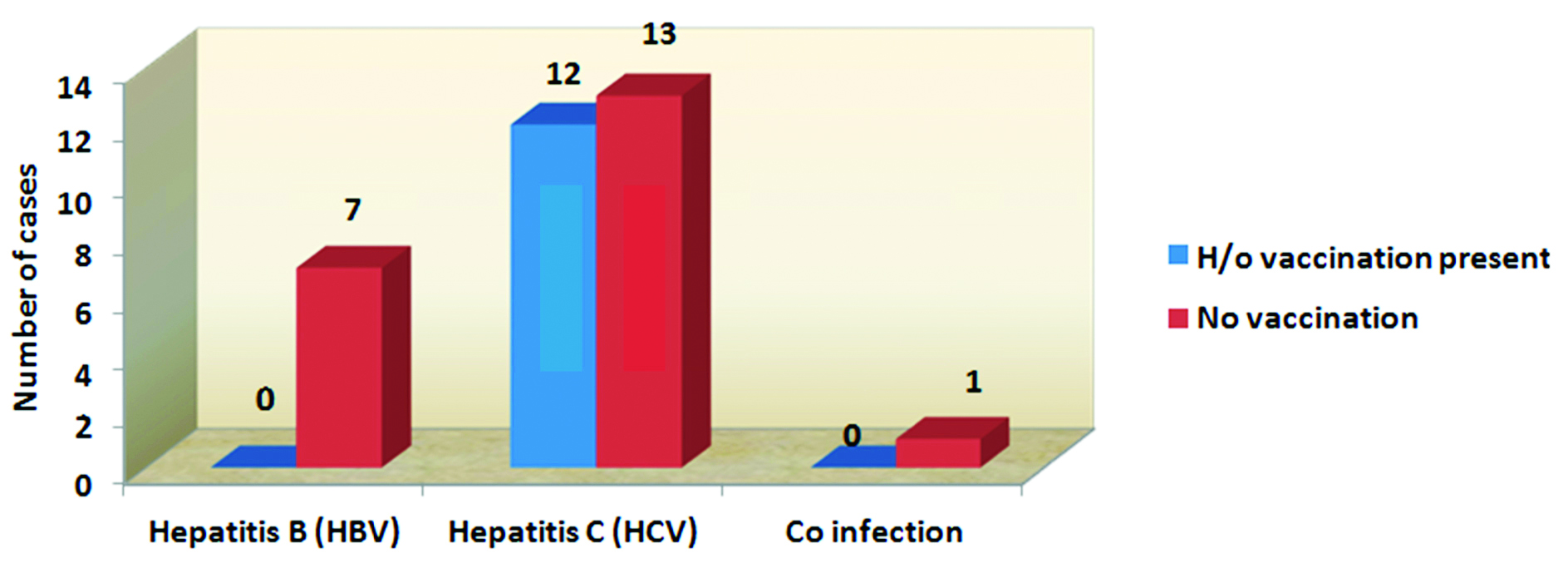
Figure 5: Association between vaccination and seroprevalence.
HCV infections are seen more in case of dialysis vintage ≥ 5years. HBV infections are seen in patients having dialysis vintage < 5years (Figure 6). The p value is < 0.02 by Yates corrected chi square test (p < 0.05 hence statistically significant).
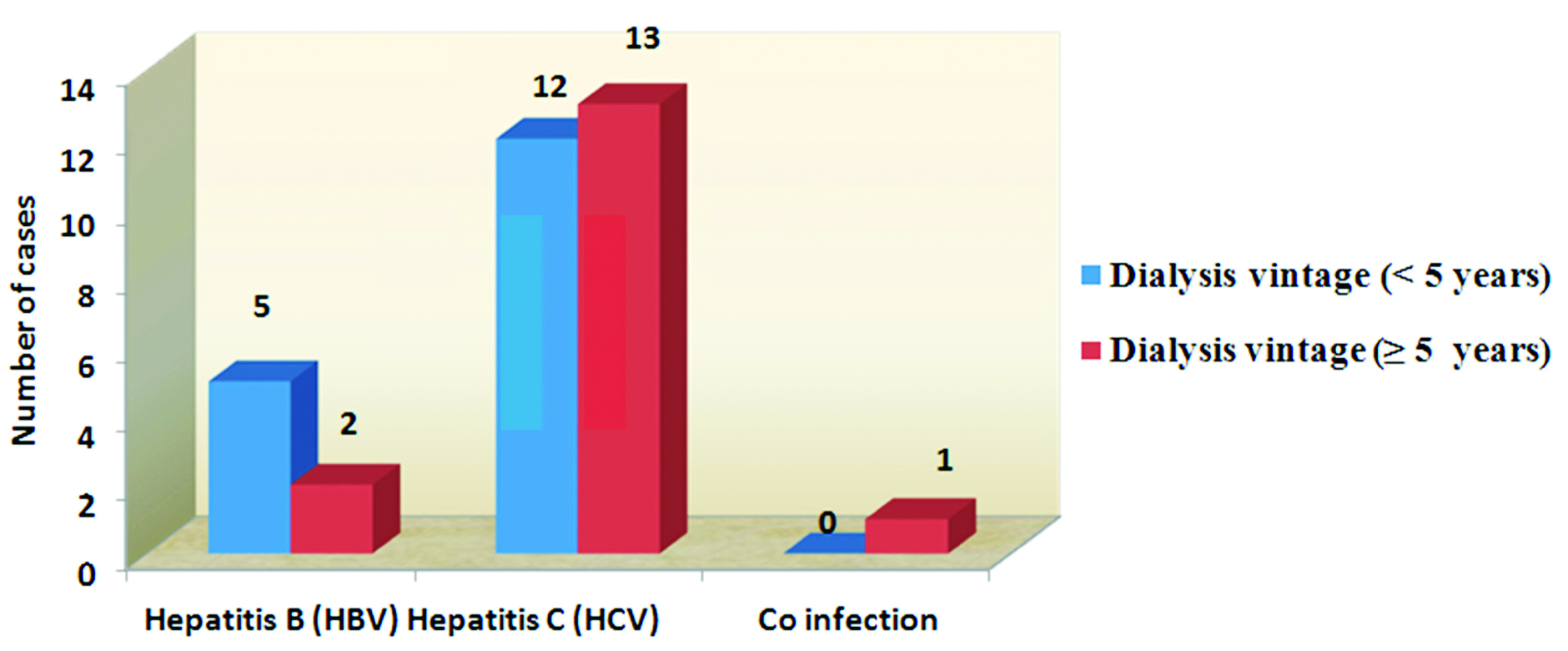
Figure 6: Association between dialysis vintage and seroprevalence.
Discussion
In the present study, the prevalence of HBV infection in HD patients was 1.84% and the prevalence of HCV infection in HD patients was 6.57%. However, the prevalence of HBV among dialysis patients in India is reported to range between 3.4–43% [18, 19]. The prevalence of HCV infection in the western countries ranges between 4 and 23.3% [20, 21]. In contrast, a study done in Asian countries found the prevalence of HBV infection to be between 1.3% and 14.6% which is similar to this study [22]. A study by Burdick et al., showed a HBV prevalence of 0–6.6% across dialysis facilities in Western Europe, Japan, and the USA [23]. HCV prevalence in HD patients varies geographically both within and between countries. Some studies have suggested a decline in HCV prevalence among HD patients in the recent years, mostly due to universal precautions. Studies by Reddy et al., and Harmankaya et al., showed the prevalence of 4.7% and 5.9%, respectively [24].
The variations in prevalence of HBV and HCV depend mainly on the strict adherence to universal infection control precautions. Adherence to strict infection control and isolation measures also will decrease the HBV and HCV prevalence rate among these patients. In the present study, HCV (6.31%) prevalence was higher than HBV (1.84%) in HD patients, which correlates with an earlier study which reported the prevalence of HCV and HBV to be 20.2% and 13.3%, respectively, in HD patients [25]. Also the seroprevalence of HCV in CKD stage V patients on hemodialysis were comparatively higher (6.57%) than seroprevalence of HCV in general population of India (3.7%).
The present study showed that history of blood transfusion is significant (p value = 0.006) in transmission of HCV infection shown in Figure 2. Prior to effective screening of blood donations, HCV infection was associated with blood transfusions needed to correct the anemia associated with kidney disease [26, 27]. Patient to patient transmission in HD units is also reported [28, 29].
The blood transfusion data of HD patients showed that HBV and HCV infected patients had received blood transfusions compared (12.5% and 50% of HBV and HCV infected patients respectively) to the uninfected patients which was contradictory to report of Natov et al., which showed more number of transfusions in HCV infected patients [30]. However, another Indian study by Salunkhe et al. had not shown any difference in this context, and hence, it is difficult to establish a correlation between the number of transfusions and the HBV/HCV infections [31]. Several studies have shown that the risk of acquiring the HCV infection increase with an increase in the number of units of blood which were transfused [32].
Vaccination offers significant protection against HBV infection. Although, vaccine response rates are low and unpredictable in dialysis patients. Because of the impaired immune response, HD patients are given larger doses of the vaccine and sometimes revaccination to produce adequate antibody titer [33]. In the present study, vaccination against HBV showed a significant association (p value = 0.04) for protection against HBV infection. Among unvaccinated patients, the prevalence of HBV is 7% and HCV is 13% (Figure 3). In the absence of a vaccine, routine screening for infection and strict adherence to standard infection control practices are vital for preventing HCV transmission in HD units [34, 35].
In the present study, dialysis vintage of > 5years showed p value = 0.02, hence statistically significant (Figure 4). In concordance with the results, the Dialysis Outcome and Practice Pattern Study (DOPPS) showed that high HCV seroconversion was associated with a longer time on dialysis, and concluded that seroconversion was associated with an increase in the facility HCV prevalence, but not with the isolation of HCV-infected patients [36].
Various observations support the theory of transmission of HCV among HD patients including increasing duration of HD, the higher incidence of HCV in units with a high prevalence of infection, and the relative homogeneity of HCV isolates in patients receiving treatment in the same HD unit [37].
Conclusion
Chronic renal failure patients who undergo repeated HD are at high risk of developing HBV and HCV. They need to be monitored for the early detection of these infections. Blood transfusions played an important role in transmission of HBV and HCV in hemodialysis patients. Duration of dialysis plays an important risk factor for infection. As longer the dialysis vintage there is more possibility of getting infected by HBV and HCV. To decrease the transmission of infection, the patients need to be segregated from the infected patients in the hospital and separate dialysis units have to be used for these infected patients. Prevention of uninfected patients with hepatitis B vaccination and treatment for HCV infected persons will decrease morbidity.
Acknowledgment
Department of Microbiology, Krishna Institute of Medical Sciences Ltd., Secunderabad, Telangana.
Conflicts of interest
Authors declare no conflicts of interest.
References
[1] Joshi R, Cardona M, Iyengar S, SukumarA, Raju CR, et al. Chronic diseases now leading cause of death in rural India–mortality data from the Andhra Pradesh rural health initiative. Int J Epidemiol. 2006; 35(6):1522–1529.
[2] Perico N, CattaneoD, Bikbov B, Remuzzi G. Hepatitis C infection and chronic renal diseases. Clin J Am Soc Nephrol. 2009; 4(1):207–220.
[3] Mohan V, Sandeep S, Deepa R, Shah B, Varghese C. Epidemiology of type 2 diabetes: Indian scenario. Indian J Med Res. 2007; 125(3):217–230.
[4] Gupta R. Trends in hypertension epidemiology in India. J Hum Hypertens. 2004; 18(2):73–78.
[5] Snyder S, Pendergraph B. Detection and evaluation of chronic kidney disease. Am Fam Physician. 2005; 72(9):1723–1732.
[6] Rajapurkar MM, John GT, Kirpalani AL, Abraham G, Agarwal SK, et al. What do we know about chronic kidney disease in India: First report of the Indian CKD registry. BMC Nephrol. 2012; 13:10.
[7] Rao M, Juneja R, Shirley RBM, Jacob CK. Haemodialysis for end stage renal disease in Southern India. A perspective from a tertiary care center. Nephrol Dial Transplant. 1998; 13(10):2494–2500.
[8] Saran R, Li Y, Robinson B, Ayanian J, Balkrishnan R, et al. US renal data system 2014 annual data report: Epidemiology of kidney disease in the United States. 2015; 66(1 Suppl 1):Svii, S1-305.
[9] Bhaumik P, Debnath K. Prevalence of hepatitis B and C among Hemodialysis patients of Tripura, India. Euroasian J Hepato-Gastroenterol. 2012; 2(1):10–13.
[10] Fabrizi F, Martin P, Dixit V, Bunnapradist S, Dulai G. Meta-analysis: Effect of hepatitis C virus infection or mortality in dialysis. Aliment Pharmacol Ther. 2004; 20(11–12):1271–1277.
[11] World Health Organization. Hepatitis C. Key facts. (accessed on 24 June 2017). http://www.who.int/en/news-room/fact-sheets/detail/hepatitis-c
[12] Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012; 380 (9859):2095–2128.
[13] Natov SN, Lau JY, BouthotBA, Murthy BV, Ruthazer R, et al. Serologic and virologic profiles of hepatitis C infection in renal transplant candidates. New England Organ Bank Hepatitis C Study Group. Am J Kidney Dis. 1998; 31(6):920–927.
[14] Cendorologo NM, Draibe SA, Silva AE, Ferraz ML, Granato C, et al. Incidence of and risk factors for hepatitis B virus and hepatitis C virus infection among hemodialysis patients and CAPD patients: Evidence for environmental transmission. Nephrol Dial Transplant. 1995; 10(2):240–246.
[15] Jeffers LJ, Perez GO, Medina MD, Ortiz ICJ, Schiff ER, et al. Hepatitis C infection in two urban hemodialysis units. Kidney Int. 1990; 38(2):320–322.
[16] Bergman S, Accortt N, Turner A, Glaze J. Hepatitis C infection is acquired pre-ESRD. Am J Kidney Dis. 2005; 45(4):684–689.
[17] Ortho-Clinical Diagnostics Pub. No. J03805; (42); 2002–2007:10–11.
[18] Sudan SS, Sharma RK. Prevalence of hepatitis B and C infection on maintenance haemodialysis. Bombay Hosp J. 2013; 45.
[19] Chandra M, Khaja MN, Hussain MM, Poduri CD, Farees N, et al. Prevalence of hepatitis B and hepatitis C viral infections in Indian patients with chronic renal failure. Intervirology. 2004; 47(6):374–376.
[20] Hinrichsen H, Leimenstoll G, Stegen G, Schrader H, Folsch UR, et al. Prevalence and risk factors of hepatitis C virus infection in haemodialysis patients: A multicentre study in 2796 patients. Gut. 2002; 51(3):429–433.
[21] Kelley VA, Everett-Kitchens J, Brannon LE, Connor K, Martinez EJ, et al. Lack of seronegative hepatitis C virus infections in patients with chronic renal failure. Transplantation. 2002; 74(10):1473–1475.
[22] Burdick RA, Bragg-Gresham JL, Woods JD, Hedderwick SA, Kurokawa K, et al. Patterns of hepatitis B prevalence and seroconversion in hemodialysis units from three continents: The DOPPS. Kidney Int. 2003; 63(6):2222–2229.
[23] Johnson DW, Dent H, Yao Q, Tranaeus A, Huang CC, et al. Frequencies of hepatitis B and C infections among haemodialysis and peritoneal dialysis patients in Asia‑Pacific countries: Analysis of registry data. Nephrol Dial Transplant. 2009; 24(5):1598–603.
[24] Harmankaya O, Cetin B, Obek A, Seber E. Low prevalence of hepatitis C virus infection in hemodialysis units: Effect of isolation? Ren Fail. 2002; 24(5):639–644.
[25] Yakaryilmaz F, Gurbuz OA, Guliter S, Mert A, Songur Y, et al. Prevalence of occult hepatitis B and hepatitis C virus infections in Turkish hemodialysis patients. Ren Fail. 2006; 28(8):729–735.
[26] Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney int. 2013; 3(1):1–150.
[27] Perumal A, Ratnam PVJ, Nair S, Anitha P, Illangovan V, et al. Seroprevalence of hepatitis B and C in patients on hemodialysis and their antibody response to hepatitis B vaccination. J Curr Res Sci Med. 2016; 2(1):20–23.
[28] Khan S, Attaullah S, Ali I, Ayaz S, Naseemullah, et al. Rising burden of Hepatitis C Virus in hemodialysis patients. J Viro. 2011; 8:438.
[29] Allander T, Medin C, Jacobson SH, Grillner L, Persson MA. Hepatitis C transmission in a hemodialysis unit: molecular evidence for spread of virus among patients not sharing equipment. J Med Virol. 1994; 43(4):415–419.
[30] Natov SN, Lau JY, Bouthot BA, Murthy BV, Ruthazer R, et al. Serologic and virologic profiles of hepatitis C infection in renal transplant candidates. New England Organ Bank Hepatitis C Study Group. Am J Kidney Dis. 1998; 31(6):920–927.
[31] Salunkhe PN, Naik SR, Semwal SN, Naik S, Kher V. Prevalence of antibodies to hepatitis C virus in HBsAg negative hemodialysis patients. Indian J Gastroenterol. 1992; 11(4):164–165.
[32] Saab S, Martion P, Brezina M, Gitrich G, Yee HFJ. Serum alanine aminotransferase in hepatitis C screening of the patients on hemodialysis. Am J Kidney Dis. 2001; 37(2):308–315.
[33] Roadby RA, Trenholme GM. Vaccination of the dialysis patient. Semin Dial. 1991; 4:102–105.
[34] Mbaeyi C, Thompson ND. Hepatitis C virus screening and management of seroconversions in hemodialysis facilities. Semin Dial. 2013; 26(4):439–46.
[35] Martin P, Fabrizi F. Hepatitis C virus and kidney disease. J Hepatol. 2008; 49(4):613–624.
[36] Benouda A, Boujdiya Z, Ahid S, Abouqal R, Adnaoui M. Prevalence of hepatitis C virus infection in Morocco and serological tests assessment of detection for the viremia prediction. Pathol Biol. 2009; 57(5):368–372.
[37] Kwon E, Cho JH, Jang HM, Kim YS, Kang SW, et al. Differential effect of viral hepatitis infection on mortality among Korean maintenance dialysis patients: A prospective multicenter cohort study. PLoS ONE. 2015; 10(8):e0135476.